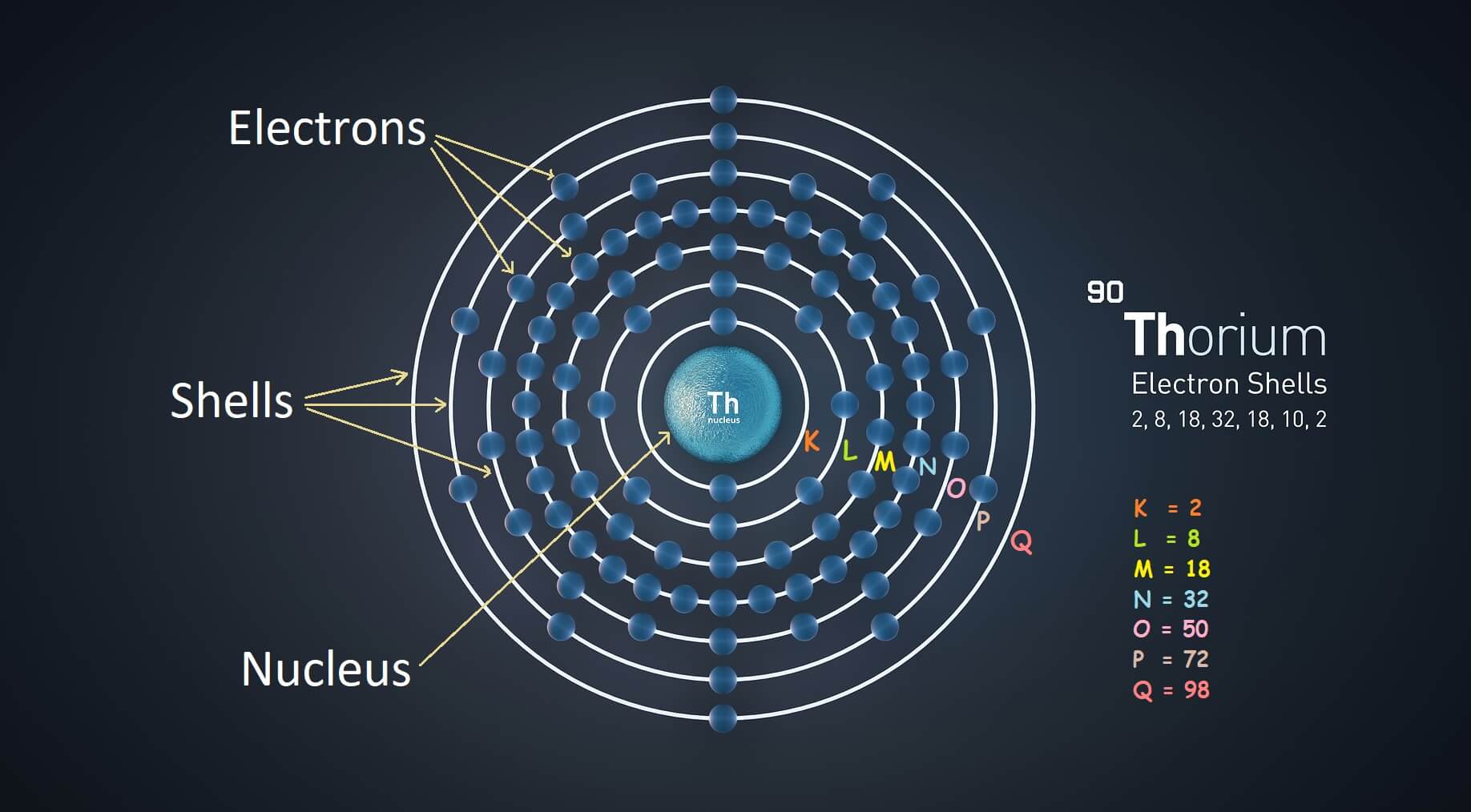
What Is Meant By Shielding Of Electrons In An Atom . Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Web shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen.
from newtondesk.com
The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. Hence, the nucleus has less grip on the outer electrons and are shielded. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Web shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons.
Electron Configuration of Elements Chemistry Periodic Table
What Is Meant By Shielding Of Electrons In An Atom Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Web shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation What Is Meant By Shielding Of Electrons In An Atom The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Web shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded. Web the concept of electron shielding, in which. What Is Meant By Shielding Of Electrons In An Atom.
From sciencenotes.org
List of Electron Configurations of Elements What Is Meant By Shielding Of Electrons In An Atom Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The nucleus of an atom contains protons, which carry positive charges, while. What Is Meant By Shielding Of Electrons In An Atom.
From www.lifeschemistrypress.com
5. The reality of electrons Life's Chemistry Press What Is Meant By Shielding Of Electrons In An Atom The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Hence, the nucleus has less grip on the outer electrons and are shielded. Web shielding. What Is Meant By Shielding Of Electrons In An Atom.
From quiznutritions.z13.web.core.windows.net
How To Calculate Shielding Electrons What Is Meant By Shielding Of Electrons In An Atom Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the. What Is Meant By Shielding Of Electrons In An Atom.
From ceyzfvbm.blob.core.windows.net
V) What Is Meant By Shielding Of Electrons In An Atom at Christina What Is Meant By Shielding Of Electrons In An Atom The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Hence, the nucleus has less grip on the outer electrons and are shielded. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of. What Is Meant By Shielding Of Electrons In An Atom.
From www.chemistrysteps.com
NMR Chemical Shift ppm, Upfield, Downfield Chemistry Steps What Is Meant By Shielding Of Electrons In An Atom Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Hence, the nucleus has less grip on the outer electrons and are shielded. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. Web shielding. What Is Meant By Shielding Of Electrons In An Atom.
From thechemistrynotes.com
Shielding effect What Is Meant By Shielding Of Electrons In An Atom The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. Web shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Web the. What Is Meant By Shielding Of Electrons In An Atom.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Is Meant By Shielding Of Electrons In An Atom The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Hence, the nucleus has less grip on the outer electrons and are shielded. Web shielding. What Is Meant By Shielding Of Electrons In An Atom.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Is Meant By Shielding Of Electrons In An Atom The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Hence, the nucleus has less grip on the outer electrons and are shielded. Web the concept of electron shielding,. What Is Meant By Shielding Of Electrons In An Atom.
From chem.libretexts.org
3.2 Shielding Chemistry LibreTexts What Is Meant By Shielding Of Electrons In An Atom The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by. What Is Meant By Shielding Of Electrons In An Atom.
From ceyzfvbm.blob.core.windows.net
V) What Is Meant By Shielding Of Electrons In An Atom at Christina What Is Meant By Shielding Of Electrons In An Atom Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Hence, the nucleus has less grip on the outer electrons and are. What Is Meant By Shielding Of Electrons In An Atom.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive What Is Meant By Shielding Of Electrons In An Atom Web shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. Web the. What Is Meant By Shielding Of Electrons In An Atom.
From cemizhaf.blob.core.windows.net
Why Is Electron Shielding Not A Factor When You Examine A Trend Across What Is Meant By Shielding Of Electrons In An Atom The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Web shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded. Web the concept of electron shielding, in which. What Is Meant By Shielding Of Electrons In An Atom.
From surfguppy.com
What is Electronegativity? What Is Meant By Shielding Of Electrons In An Atom The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by. What Is Meant By Shielding Of Electrons In An Atom.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements What Is Meant By Shielding Of Electrons In An Atom The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Hence, the nucleus has less grip on the outer electrons and are shielded. Web the. What Is Meant By Shielding Of Electrons In An Atom.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is Meant By Shielding Of Electrons In An Atom Hence, the nucleus has less grip on the outer electrons and are shielded. Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Web shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The shielding effect describes. What Is Meant By Shielding Of Electrons In An Atom.
From spmkimia.onlinetuition.com.my
Susunan Elektron di Dalam Atom Nota Ulangkaji Kimia SPM Tingkatan 4 What Is Meant By Shielding Of Electrons In An Atom Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Web shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded. Web the concept of. What Is Meant By Shielding Of Electrons In An Atom.
From wisc.pb.unizin.org
M7Q8 Core and Valence Electrons, Shielding, Zeff Chem 103/104 What Is Meant By Shielding Of Electrons In An Atom Web the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. Hence, the nucleus has less grip on the outer electrons and are shielded. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner. What Is Meant By Shielding Of Electrons In An Atom.